How Philly Doctors Are Saving the World
Ebola. Malaria. AIDS. The weapons to fight deadly disease all around the globe are being forged right here, by the city’s brightest minds.
For our latest Top Doctors cover story, we went beyond the doctor’s office to the labs where Philadelphia-based researchers work to eradicate diseases that claim millions of lives each year. To read about their bold advances, scroll down or use these links to jump to a specific topic:
- Ebola: Fear’s Front Line
- AIDS: Modern Warfare
- Cancer: A New View
- Pancreatic Cancer: An Unlikely Weapon
- Hepatitis: The (Other) Big C
- Malaria: A Moving Target
- The Warriors: Philly Doctors Around the Globe
- The Vaccine Problem: Solved
- Show Me The Money: What Research Should Be Funded Next
Ebola: Fear’s Front Line
Taking on a terrifying disease, overseas and at home. By Sandy Hingston

Trish Henwood, director of global health initiatives for Penn’s Emergency Medicine department. Photograph by Jonathan Pushnik
In March, the world health organization estimated that the most recent outbreak of Ebola had killed more than 10,000 West Africans. Trish Henwood, director of global health initiatives for Penn’s Emergency Medicine department, saw too many of those deaths firsthand on trips last fall and winter with the International Medical Corps to treat the infected in Liberia. Parents lost children; children were orphaned. The acrid scent of disinfecting chlorine was everywhere. Doctors and nurses were decked out in ghostly gear and goggles to prevent contact with their patients’ bodily fluids. “The level of fear,” Henwood says, “couldn’t have been higher.”
Before this latest — and deadliest-ever — epidemic began, Thomas Jefferson University biomedical researcher Matthias Schnell says, drug companies weren’t interested in an Ebola vaccine: “There’s not a lot of long-term planning for these diseases. We go from panic to doing nothing.” He’s convinced, though, that vaccines are our best defense against Ebola. Several are now in development, including one Schnell has been working on: a set of two shots of a serum that protects against both Ebola and rabies.
There are challenges to vaccinating in the developing world, he notes. It’s hard to keep vaccines temperature-stable (see below), to get patients to agree to long-term monitoring, for them to come back for the multiple shots required. There are home-turf challenges as well: “The market for vaccines isn’t that great, because they work,” says Schnell. “In the U.S., all our vaccines put together make the same money each year as Lipitor” — a single cholesterol drug.
Schnell is seeking approval from the FDA to trial his vaccine in humans, and is looking into ways to produce the antibodies it creates in large enough amounts to treat those who’ve already been infected with Ebola. Like all the researchers we spoke to for this Top Doctors issue, he said funding is a constant concern. The one certainty about Ebola is that it will come back. “The situation is under control in West Africa now,” Schnell points out. “But Ebola wasn’t a problem in West Africa for a long time. It was in Sudan and Uganda and Congo.”
When it does return, Henwood and the local on-site workers she lauds — they had to brave not only the disease, she says, but also stigmatization by their communities — will do their best to meet the challenge. The competitive triathlete says, “You have to embrace the sweat. It helped me to have that perspective.” What doesn’t help? Fear-fanning by the media, who, she says, “never focus on the science, just the worst-case scenarios.” The lesson to be drawn from this round of the disease: “With travel and technology, the world is a really small place. Things that seem isolated in West Africa are not so distant. We need to help these nations strengthen their health systems, because they will affect us locally.”
AIDS: Modern Warfare
New artillery aims at an end to the HIV/AIDS epidemic. By Emily Leaman
HIV particles bud from a white blood cell. Photograph from the CDC
The Brains: Kamel Khalili and Wenhui Hu, Temple University School of Medicine
The Goal: Eliminate HIV from human cells
The Attack: This proof-of-concept therapy could result in a permanent cure for AIDS and protection against HIV. Using a DNA-snipping enzyme and a strand of RNA, the researchers were able to edit HIV-1 DNA out of the genetic code of cultured human cells, leaving them virus-free. The next frontier: a delivery system that works on all infected cells and all mutations of the virus.
The Brain: Jeffrey Jacobson, Drexel University College of Medicine
The Goal: Design a better treatment for HIV/AIDS
The Attack: Jacobson is leading clinical trials for an injectable once-a-week drug that dispatches antibodies to block a cell’s HIV receptor points, keeping the virus out of healthy cells and preventing it from replicating. The therapy replaces a burdensome daily regimen of antiretroviral pills and is less toxic to boot, since it leverages a molecule produced by the body itself.
The Brains: Irwin Chaiken and Cameron Abrams, Drexel University College of Medicine and Drexel University College of Engineering
The Goal: Trick the virus into destroying itself
The Attack: In a lab study, the pair have developed a molecule that binds to the surface of HIV and hijacks the virus’s machinery. The virus encounters this molecule — which it mistakes for a healthy cell — and sheds a protective protein. Without the protein, the virus deflates like a balloon before it can cause any damage. The next step: showing that the molecule is viable in living creatures.
The Brains: Carl H. June, Bruce L. Levine and Pablo Tebas, University of Pennsylvania
The Goal: Reengineer cells to make them resistant to HIV infection
The Attack: In a study of 12 HIV-positive patients, the team was able to induce a mutation in T-cells to lock out HIV, rendering patients’ cells resistant to infection. Researchers are now working with a larger pool of subjects in hopes the therapy could be a functional cure for HIV/AIDS.
The Brain: Philip R. Johnson, Children’s Hospital of Philadelphia
The Goal: Develop an HIV vaccine using gene therapy
The Attack: In 2009, Johnson used monkeys to develop a gene therapy that protected the animals from SIV, the primate version of HIV. Researchers created artificial genes that produce SIV antibodies, loaded the genes into viruses, and injected the viruses into monkeys, prompting the monkeys’ cells to begin producing antibodies. The result: an effectual SIV vaccine. Johnson’s method has entered early-stage human trials in the U.K.
Cancer: A New View
These high-tech goggles are changing the way we see cancer — literally. By Adjua Fisher

Illustration by Bryan Christie Design
When surgeons remove cancerous tumors, they rely on what they can see and feel. Because they can’t see microscopically, sometimes they don’t excise all of a tumor in the first round of surgery. That’s where these goggles, currently being trialed at the Lankenau Institute for Medical Research, come in.
First, doctors inject a special dye into the patient’s blood that latches onto the abnormal proteins on the surface of the cancerous cells. Then, during surgery, the goggles’ detection screen picks up the dye in the body; the cancer cells glow blue and create a virtual map, letting surgeons see cells they would otherwise miss and remove them all in one fell swoop. The patient avoids multiple surgeries, and there’s less risk a fast-moving cancer will spread during recovery.
Lankenau clinical research director Gerald Messerschmidt refers to the goggles, created by researchers at the Washington University School of Medicine in St. Louis, as “Superman eyes.” They’re still in the experimental stage, with limited use. But one day, he hopes, they’ll be used in operating rooms around the world. “There are many countries today where if you have cancer, treatments are limited due to resources,” he says. “These goggles could make a big difference.”
Pancreatic Cancer: An Unlikely Weapon
Has a deadly cancer met its match? By Sandy Hingston

Timothy J. Yen, a cancer researcher at Fox Chase Cancer Center. Photograph by Jonathan Pushnik
Nobody wants cancer, but you really don’t want pancreatic cancer; 95 percent of those diagnosed die within five years. That’s partly because symptoms don’t appear until the disease is far advanced, but also because it doesn’t respond well to chemotherapy. Now, Timothy J. Yen, a cancer researcher at Fox Chase Cancer Center, has shown why.
Yen and his lab team tested each of the 24,000 genes in pancreatic cancer cells to find which caused their resistance to gemcitabine, a common chemo drug that inhibits DNA replication so cells can’t divide. To their surprise, the culprit was the vitamin D receptor, or VDR, which works in normal cells to promote inflammatory responses, detoxification and even hair growth. The cancer cells were hijacking these receptors and repurposing them to repair the damage caused by gemcitabine.
It wasn’t what Yen or anyone else expected. “I said, ‘I’ll play along. Show me!’” he recalls. Lab technicians then compared the action of drugs known to destroy cancer cells with what happened when they knocked out the vitamin D receptor. The latter method was just as effective, and worked across several different pancreatic cancer lines. “I became more of a believer,” says Yen.
The cancer cells, he adds, are dependent on VDR to survive, but normal cells aren’t: “So we can hammer away at the VDR for the short time-span of treatment.” And the method may work against other so-called “recalcitrant” cancers — ovarian, brain, lung — that also survive chemo drugs. “The concept is generally applicable,” says Yen. “Now we have a mechanism.” Still ahead: animal and then, someday, patient studies.
Hepatitis: The (Other) Big C
A cure for an under-the-radar killer. By Emily Leaman

Rajender Reddy, director of hepatology at Penn Medicine. Photograph by Jonathan Pushnik
Imagine walking around for years with a chronic, potentially deadly disease you didn’t know you had. Decades after being infected, you begin showing signs of serious liver damage: bloody vomit, itchy skin, a swollen abdomen, bruises. Respiratory issues may emerge, and liver cancer isn’t out of the question. Eventually, you need a liver transplant; without it, you’ll die.
Now imagine finding out the infection that caused the disease was curable — if you’d discovered it sooner. “Hepatitis C is the silent epidemic,” says Rajender Reddy, director of hepatology at Penn Medicine and a leader in hep C treatment. “Because it may be totally asymptomatic, the majority of people don’t seek medical attention.”
Since the early 1990s, treatments have been available for the viral infection, which is primarily blood-borne. But cure rates were so low — and side effects so taxing — that many sufferers chose to forgo treatment. Besides, the regimen required weekly injections for up to 16 months — a barrier many patients couldn’t (or wouldn’t) overcome.
Now, thanks to Reddy and a team of international researchers, there’s a better option: a new cocktail of drugs that wages a full-scale attack on the virus by invading it, interrupting its ability to replicate, and ultimately eradicating it altogether. It can cut treatment time to 12 weeks and is easier for patients to bear, consisting of pills instead of injections and causing fewer, less severe side effects. The end result: an effective cure — yes, cure — in about 95 percent of patients in the earlier stages of the disease, including those for whom previous options haven’t worked.
Reddy says the next step is making the treatment more practical by lowering the cost; right now, it runs between $85,000 and $95,000, causing some insurers to balk. He also hopes to fine-tune the drugs to cut treatment time to as little as four weeks and make them effective against all genotypes of the virus. Someday, perhaps, there’ll be a hep C vaccine.
“For years I told patients, ‘We’re working on better treatments — maybe next year,’” he says. “But now I can go back and tell them the cure they’ve been hoping for is finally here. It’s very gratifying.”
Malaria: A Moving Target
As the disease adapts, science does, too. By Sandy Hingston

Akhil Vaidya, a molecular biologist at Drexel University College of Medicine. Photograph by Jonathan Pushnik
If god made you his helper,” Akhil Vaidya says, “and told you to design a creature, and you showed Him this” — he points to his computer screen, which displays the life stages of Plasmodium falciparum — “He would laugh.” The parasite causing malaria in humans has a comically complicated existence: It enters a human through a bite from an infected female mosquito, invades the liver, replicates asexually, invades red blood cells and bursts them (causing fever and chills), invades more red blood cells, divides into male and female gametocytes, one each of which must be ingested by yet another female mosquito biting through human skin …
As unwieldy as this seems, there are millions of cases of malaria each year, and more than a million deaths, mostly of children and pregnant women. There are drugs to fight it, but the best one, artemisinin, is losing its potency in Southeast Asia. “If that resistance spreads, we’ll be in deep trouble,” Vaidya says. “It’s a constant battle. The more you push an organism, the more it will try to evolve.”
For 50 years, Vaidya and his fellow molecular biologists at Drexel University College of Medicine (and its predecessor, Hahnemann) have been studying malaria. They still don’t know how the drugs that fight it — artemisinin, quinine, chloroquine — work. But Vaidya has discovered a new class of chemical compounds, pyrazoleamides, that increase sodium levels in the parasites in infected red blood cells, causing them to swell and rupture. A similar drug is being developed by Novartis. Competition? Vaidya shrugs. The more weapons against this ever-evolving enemy, the better. Work is proceeding on malaria vaccines, but Plasmodium’s complex life cycle makes that a hard slog, too. Pharma companies support malaria research, Vaidya says wryly, “but not like they support drugs for erectile dysfunction and baldness.”
At Penn, microbiologist Beatrice Hahn is attacking malaria from the opposite direction. Her lab has traced the parasite to the Western lowland gorilla population that first transmitted it to humans. On her computer, she pulls up elegant color-coded family trees generated by analysis of the genetic sequences in gorilla and chimpanzee feces from Africa, where the disease originated. The samples were left over from the lab’s similar work on HIV and its chimp precursor, SIV. Cross-species transmissions of pathogens are rare, Hahn explains; in order to generate a recombinant virus that could infect humans, a chimp had to have two concurrent infections. “There are good barriers in place to prevent such transmission,” says Hahn. “But these microbes find holes in the armor. If you study them, you also find holes in the armor.” And that could be helpful when the next deadly enemy makes its leap.
The Warriors: Philly Doctors Around the Globe
From delivering wheelchairs to delivering babies, these Philly medical pros — and thousands more like them — work the front lines, tackling medical crises all over the world. By Adjua Fisher

From left: Yanick Vibert, Rodger Barnette, Veronica Hache, Scott Kozin, Javad Parvizi, and Matt Fields. Photograph by Jonathan Pushnik
Yanick Vibert, St. Chirstopher’s Hospital for Children
For 20 years, this neonatologist has traveled to developing countries like Haiti, Guyana and Uganda to train health-care workers to recognize and treat respiratory distress in newborns.
Rodger Barnette, Temple University School of Medicine
In July, the chair of Temple’s anesthesiology department embarks on a five-year mission to Kenya to teach and supervise medical students in anesthesiology and treat patients in an intensive-care unit.
Veronica Hache, Springfield Hospital
For the past decade, this physical therapist has gone on regular missions to developing countries in South America to deliver wheelchairs to the disabled.
Scott Kozin, Shriners Hospitals for Children
This surgeon and chief of staff founded the Touching Hands Project, which goes on worldwide missions to treat hand and upper-extremity ailments in those without access to medical care.
Javad Parvizi, Rothman Institute
A surgeon and world leader in joint research, Parvizi led an international effort to create a comprehensive joint-infection bible that’s now published in a dozen-plus languages and used around the globe.
Matt Fields, Thomas Jefferson University Hospital
Fields, an ultrasound expert, travels to Sierra Leone to teach physicians how to use portable ultrasound machines to diagnose often-fatal health problems that would otherwise be missed.
The Vaccine Problem: Solved
By Emily Leaman
According to the world Health Organization, 1.5 million children die each year from common vaccine-preventable diseases. It’s not because there aren’t enough vaccines to go around. The problem lies in keeping the lifesaving antigens cool enough — between two and eight degrees Celsius, to be exact — to remain viable, an especially difficult task in parts of the world where electricity can be hard to come by. Up to 40 percent of vaccines spoil due to improper storage.
Five years ago, Penn medicine and microbiology professor Harvey Rubin realized the answer to this gargantuan global health-care problem was actually quite simple: Use power from off-grid cell-phone towers, which are ubiquitous the world over and largely immune to power outages, to run refrigerators that store vaccines.
Through his nonprofit organization, Energize the Chain, Rubin has partnered with local wireless service providers and established 111 vaccine-storage sites in Zimbabwe — with another 150 in development — and is laying the groundwork for sites in Ghana and India. The result: more than 250,000 kids successfully vaccinated since 2013.
Here’s an inside look at a vaccine’s new and improved journey from lab to faraway patient:
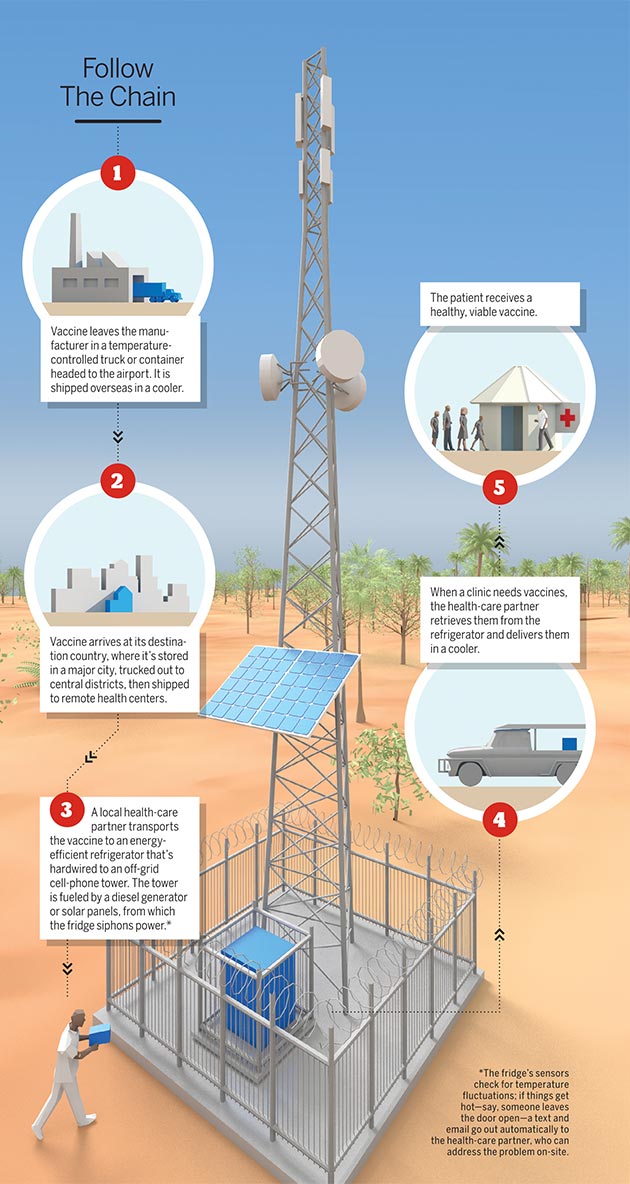
Illustration by Bryan Christie Design
Show Me The Money
Five of Philly’s top health-care minds tell how they’d allocate $100 million in research funds if it fell into their laps. By Adjua Fisher
Michael Parmacek, Department of Medicine chair, Penn Medicine: “It’s tempting to say cancer, but $100 million isn’t going to cure cancer. This century’s disease epidemic is diabetes. I’d launch a multi-pronged attack on diabetes, starting at its root causes by investing in nutrition research and then focusing on novel therapies beyond insulin.”
Joseph St. Geme, Department of Pediatrics chair, CHOP: “I’d develop groups of ‘health-care extenders’ — communityleaders who promote basic medical care in developing countries. These leaders could help tackle the major preventable problems that kill tens of thousands of children daily, including malnutrition, diarrhea, pneumonia and malaria.”
Daniel V. Schidlow, dean, Drexel University College of Medicine: “I would address anti-microbial resistance — the loss of effectiveness of drugs that treat bacterial, viral, parasitic and fungal infections. AMR has emerged as a worldwide threat to the treatment of major causes of death — malaria, HIV, tuberculosis — and causes significant damage to economic development and sustainability.”
Herbert Cushing, chief medical officer, Temple University Hospital: “Funding should be split among improving economic conditions in developing countries; spreading the capacity to implement known solutions to high-impact conditions like diarrheal and respiratory illness and infections like HIV/AIDS, tuberculosis and malaria; and ramping up practices to address emerging infections.”
Anne Docimo, chief medical officer, Jefferson Health System: “The World Health Organization estimates that one in 10 people worldwide die from smoking-related causes, and smoking is a key underlying factor in heart disease, stroke and lung disease. Investing in smoking cessation and prevention worldwide would dramatically reduce deaths.”
Originally published in the May 2015 issue of Philadelphia magazine.


